ALS offers bioaccessibility testing developed to replicate human stomach and intestines which in turn provides an assessment of the bioaccessible share of metals in contaminated soil after oral intake.
Testing the bioaccessibility will increase the precision of the health risk assessment of a particular site and help put in the right remediation efforts.
Bioaccessibility as part of risk assessment of contaminated soil
In an area where oral intake of soil constitutes an important exposure factor, for example where small children are present, a bioaccessibility test can be useful to increase the precision of the health risk assessment of a site.
A risk assessment based solely on total metal concentrations may result in an overestimation of the risks. An improved risk assessment facilitates applying the right remediation measures.

The method
This in-vitro test offered by method UBM ISO-17924:2018. The metals arsenic (As), cadmium (Cd) and lead (Pb) are routinely measured but ALS offers analysis of about 60 additional metals.
According to the Unified BARGE Method (UBM) a representative soil samples is exposed to saliva, gastric extractant, bile and intestinal extractant in a sequential leaching to simulate the physiological environment of the human digestive system.
To further recreate the physiological conditions, the test is conducted under the body’s natural temperature and pH simulating intestinal movement. The leaching is performed in two stages:
- Saliva and gastric extractant
- Intestinal extractant and bile
The solutions from each step are analyzed separately. Please see image below.
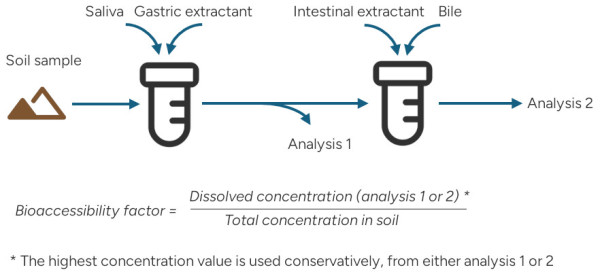
Metal concentrations are reported as mg/kg from each leaching test. The bioaccessibility factor is calculated based on the highest concentrations from leaching 1 or 2, divided by sample total metal concentrations. Total concentrations are analyzed by ICP-MS after aqua regia digestion.
Would you like to know more?
Please contact us using the form or by e-mailing us at salesspecial.lu@alsglobal.com